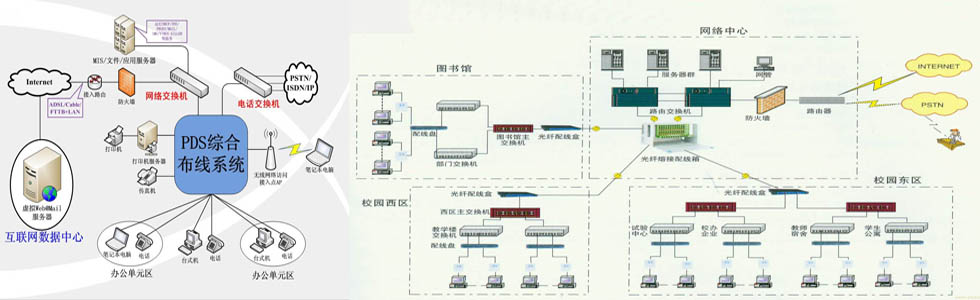
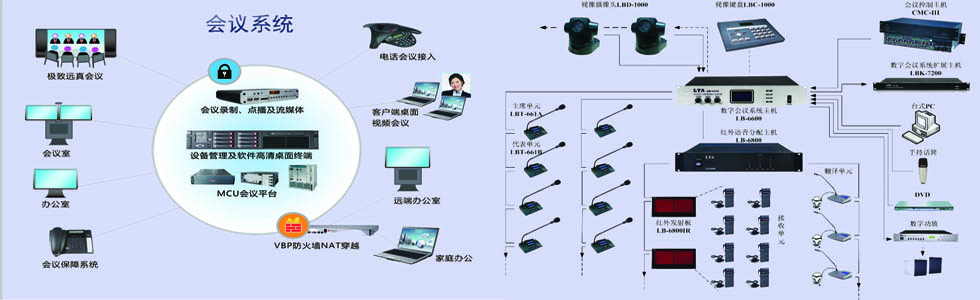
-
2020/4/14怎么选择家庭智能安防系统 家庭智能安防系统选购技巧2020/4/14海康威视发布超容量网络存储设备2020/4/14你的安防监控网络安全吗?2019/12/4安防+AI”成基调 家庭安防市场不可小觑2019/12/4海康威视发布支持Wi-Fi/4G无线连接的摄录一体2019/9/24监控摄像机是安防时代的利器2019/9/24高空监控拍照取证 违建,你被无人机“盯”上了2019/7/9家用监控摄像机哪种牌子好? 监控摄像头排行榜
-
2015/4/16相关资质
乐竞体育平台是集技术开发、产品代理销售、工程服务于一体的综合性高科技企业。前身是广州乐派数码科技有限公司系统集成部,随业务不断扩大发展,特与湖南创想伟业科技发展有限公司组成战略合作伙伴,设立乐竞体育平台。
...详细>>
...详细>>
-
2020/2/1846寸液晶大屏拼接屏走线注意事2020/2/18海康威视发布超容量网络存储设备2020/2/18小型家庭安防监控系统怎么安装【2020/2/18别墅安防系统解决方案有哪些 别2020/2/18如何维护安防视频监控 安防视频
-
2021/7/21多媒体教室解决方案2020/2/18智能家居如何防盗 无线遥控五步2020/2/18海康威视综合体综合安防解决方案2019/7/9电梯无线监控方案2019/7/9海康威视发布低功耗摄像机系统解2019/7/9高空瞭望摄像机监控系统应用方案2019/7/9智能家居安防设备如何选择 智能